When chlorine is added in its gaseous form or as a gas dissolved in water to the solution of sodium. What is a double replacement displacement reaction.
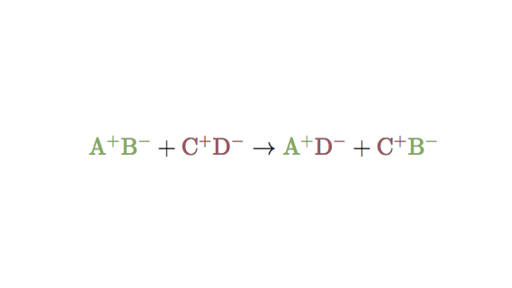
Double Replacement Reactions Double Displacement Article Khan Academy
A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products.
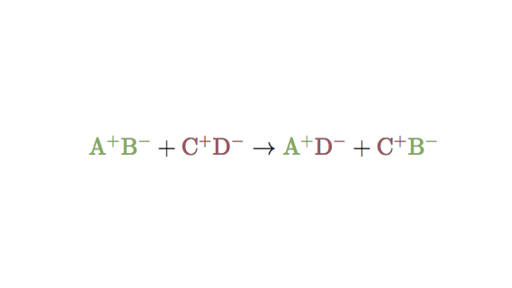
. During double replacement the cations and anions of two different compounds switch places. A type of reaction in which the ions of two compounds exchange places in an aqueous solution to form two new compounds Describe the nature of the reactants and products in a single replacement reaction. A single displacement reaction which is also called as single replacement reaction is a kind of oxidation-reduction chemical reaction when an ion or element moves out of a compound ie one element is replaced by the other in a compound.
In a single replacement reaction one of the reactants is more reactive than the other which results in the formation of a product that is more stable. Solubility rules are used to predict whether some double -. Double replacement sometimes referred to as double displacement reactions are when parts of ionic compounds are switched to form two new ionic compounds.
A double replacement reaction is a type of chemical reaction. The primary difference between a single and double replacement reaction is that in a single replacement reaction a free element is exchanged for another to yield a new compound and a new element. A double displacement reaction is also called a double replacement reaction salt metathesis reaction or double decomposition.
A double-replacement reaction exchanges the cations or the anions of two ionic compounds. What is a double replacement. Two compounds react to form two new compounds.
The name describes the process very well. In double replacement reactions the positive ions exchange negative ion partners. AgNO3 KI KNO3 AgI A.
A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative. A double replacement. Double-replacement reactions can form precipitates gases or molecular compounds.
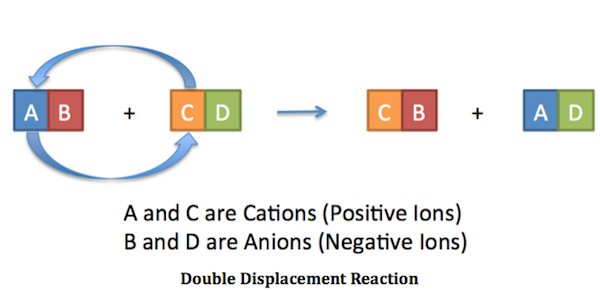
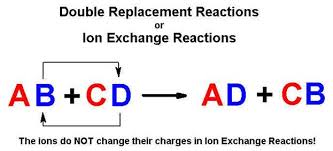
In a double replacement reaction the components of two compounds are exchanged or displaced to form two new compounds. The way I think of it since were dealing with ionic compounds is that when I write out a reaction I begin like this. The figure below clearly illustrates how this swap takes place.
You can think of the reaction as swapping the cations or the anions but not swapping both since you would end up with the same substances you started with. One compound reacts to form two separate elements. The following is a real life example of double replacement reactions.
Generally double-replacement reactions involve the exchange. What Is a Double-Replacement Reaction. The truefalse questions in this worksheet will help students review the process of a double-replacement reaction.
Select two compounds above and this calculator will predict whether or not the reaction will occur in water. Two elements reacting to form a single compound. A double replacement reaction is represented by.
Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. Single replacement 2 See answers Advertisement Advertisement thescarletspider101 thescarletspider101 Answer. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions.
AB XY --- AY XB. A precipitation reaction is a double - replacement reaction in which one product is a solid precipitate. A double-replacement reaction occurs when parts of two ionic compounds are exchanged making two new compounds.
Double replacement reactions also result in the formation of a solid product which is called a precipitate. The following is the initial neutralization reaction. The name describes the process very well.
Two replacements take place in. Written using generic symbols it is. CuCl 2 aq 2 AgNO 3 aq CuNO 3 2 aq 2 AgCls.
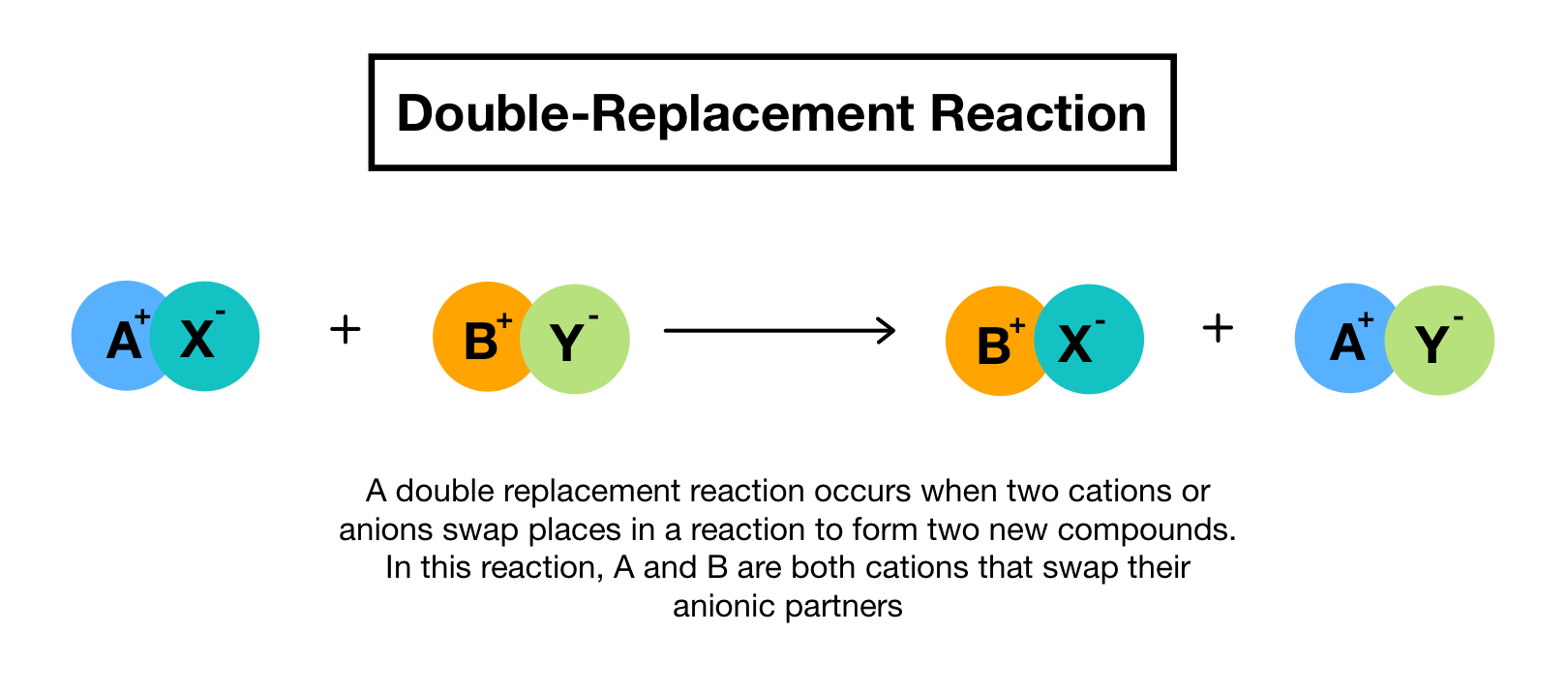
Combining vinegar and baking soda to create homemade volcano. A and X are the cations postively-charged ions in this example with B and Y being the anions negatively-charged ions. Here is another way to look at the above generic example.
In this printable students also use an equation to answer questions about elements and chemical reactions. Occasionally a reaction will produce both a gas and a molecular compound. In double replacement reactions the elements get replaced in both the reacting compounds.
The solvent for a double replacement reaction is usually water and the reactants and products are usually ionic compoundsbut they can also be acids or bases. Double replacement reaction occurs when the cations and anions of two ionic compounds are exchanged. A double replacment is a way of forming a new homogenous mixture.
Chemistry Middle School answered What type of reaction is displayed below. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. Many double displacement reactions occur between ionic compounds that are dissolved in water.
The reaction occurs most often between ionic compounds although technically the bonds formed between the chemical species may be either ionic or covalent in nature. A double replacement reaction will occur if a formation of a precipitate gas or water takes place. A double-replacement or double-displacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds.
This is simply based on the solubility chart of inorganic compounds. Stay tuned with BYJUS to learn more about other concepts such as displacement reactions.

Single Replacement Reaction Definition And Examples
How Do You Balance Double Replacement Reactions Socratic

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Double Replacement Double Displacement Reaction

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Double Replacement Reaction Definition And Examples
0 comments
Post a Comment